Appropriate Patients
Dosing


The ASCERTAIN crossover trial was designed to assess systemic decitabine exposure, demethylation activity, and safety between IV decitabine and INQOVI tablets in a broad range of patients with cytogenetic risks, including poor (24%-25%), intermediate (20%-28%), and better (39%-45%). 35% of patients had TP53 mutations, a hallmark of treatment-resistant MDS. The trial allowed for intrapatient comparison in the first 2 randomized treatment cycles, then assessment of the long-term efficacy and safety of INQOVI as a single arm.1-4
The Phase 3 crossover trial had a median follow-up of approximately 2.6 years.1,2
Phase 3 crossover design1,2
Open-label, randomized, 2-cycle, 2-sequence, crossover clinical trial in treatment-experienced or treatment-naïve patients with MDS, including CMML (IPSS intermediate-1, -2, or high-risk). Patients were allowed to have 1 prior cycle of decitabine or azacitidine, and there was no limit for body weight or surface area.
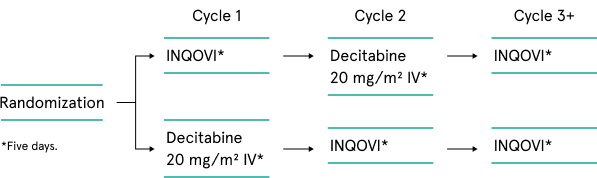
| Phase 3 (N=133)1,2 |
Phase 3 long-term follow-up (N=133)2 |
|
|---|---|---|
| Primary endpoint | 5-day area under the curve (AUC) between oral decitabine-cedazuridine and IV decitabine for Cycles 1 and 2 |
5-day area under the curve (AUC) between oral decitabine-cedazuridine and IV decitabine for Cycles 1 and 2 |
| Key secondary endpoints |
Complete response Rate of conversion from transfusion dependence to transfusion independence |
Clinical response, transfusion independence, median overall and leukemia-free survival (LFS), safety, and pharmacodynamics |
| Other endpoints |
Median duration of complete response and best response Median time to complete response |
Median duration of complete response and best response. Median time to first response |
CMML= chronic myelomonocytic leukemia; IPSS=international prognostic scoring system; MDS=myelodysplastic syndromes.
| Characteristic | Phase 3 (N=133)1,2 |
|---|---|
| Age (years) | |
| Median (min, max) | 71 (44, 88) |
| Sex | |
| Male | 65% |
| Female | 35% |
| Race | |
| White | 91% |
| Black or African-American | 3% |
| Asian | 2% |
| Other or not reported | 4% |
| ECOG performance score | |
| 0 | 41% |
| 1 | 59% |
| 2 | 0 |
| Disease category/IPSS | |
| MDS intermediate-1 risk | 44% |
| MDS intermediate-2 risk | 20% |
| MDS high risk | 16% |
| MDS low risk | 8% |
| CMML | 12% |
| Prior HMA therapya | |
| Prior azacitidine | 5% |
| Prior decitabine | 3% |
| Transfusion dependenceb | |
| RBC transfusion dependence | 39% |
| Platelet transfusion dependence | 8% |
aOne cycle only, per the exclusion criteria.
bDefined as documentation of ≥2 units of transfusion within 56 days of the first day of study treatment.
CMML=chronic myelomonocytic leukemia; ECOG=Eastern Cooperative Oncology Group; MDS=myelodysplastic syndromes; RBC=red blood cell.
*Excludes data from some participants due to data confidence or quality issues.
†Based on International Working Group 2006 myelodysplastic syndromes response criteria.
| Phase 3 (N=133) |
Phase 3 long-term follow-up (N=133) |
|
|---|---|---|
| Median follow-up time | 12.6 months (range: 9.3-20.5) |
~32 months (IQR: ~30-35) |
| Patients who achieved CR (CI)a | 21% (95% CI: 15, 29) | 25% (95% CI: 17, 34)b |
| Median duration of CRc | 7.5 months (range: 1.6-17.5) | 14.1 months (range: 11.7-18.7) |
| Median time to CRa | 4.3 months (range: 2.1-15.2) | 4.5 months (range: 2.1-18.7)5 |
aComplete or partial response may take longer than 4 cycles.1
bOf the evaluable 117 participants, 25% (29/117) achieved a complete response (CR).2
cFrom start of CR until relapse or death.1
Adverse reactions reported in ≥10% of patients in the pooled Phase 2 and Phase 3 safety population1
| Adverse reactionsa | INQOVI Cycle 1 n=107 |
IV decitabine Cycle 1 n=106 |
INQOVI all cycles n=208c |
|||
|---|---|---|---|---|---|---|
| All grades (%) |
Grades 3‑4 (%) |
All grades (%) |
Grades 3‑4 (%) |
All grades (%) |
Grades 3‑4 (%) |
|
| General disorders and administration site conditions | ||||||
| Fatigueb | 29 | 2 | 25 | 0 | 55 | 5 |
| Hemorrhageb | 24 | 2 | 17 | 0 | 43 | 3 |
| Edemab | 10 | 0 | 11 | 0 | 30 | 0.5 |
| Pyrexia | 7 | 0 | 7 | 0 | 19 | 1 |
| Gastrointestinal disorders | ||||||
| Constipationb | 20 | 0 | 23 | 0 | 44 | 0 |
| Mucositisb | 18 | 1 | 24 | 2 | 41 | 4 |
| Nausea | 25 | 0 | 16 | 0 | 40 | 0.5 |
| Diarrheab | 16 | 0 | 11 | 0 | 37 | 1 |
| Transaminase increasedb | 12 | 1 | 3 | 0 | 21 | 3 |
| Abdominal painb | 9 | 0 | 7 | 0 | 19 | 1 |
| Vomiting | 5 | 0 | 5 | 0 | 15 | 0 |
| Musculoskeletal and connective tissue disorders | ||||||
| Myalgiab | 9 | 2 | 16 | 1 | 42 | 3 |
| Arthralgiab | 9 | 1 | 13 | 1 | 40 | 3 |
| Respiratory, thoracic, and mediastinal disorders | ||||||
| Dyspneab | 17 | 3 | 9 | 3 | 38 | 6 |
| Coughb | 7 | 0 | 8 | 0 | 28 | 0 |
| Blood and lymphatic system disorders | ||||||
| Febrile neutropenia | 10 | 10 | 13 | 13 | 33 | 32 |
| Skin and subcutaneous tissue disorders | ||||||
| Rashb | 12 | 1 | 11 | 1 | 33 | 0.5 |
| Nervous system disorders | ||||||
| Dizzinessb | 16 | 1 | 11 | 0 | 33 | 2 |
| Headacheb | 22 | 0 | 13 | 0 | 30 | 0 |
| Neuropathyb | 4 | 0 | 8 | 0 | 13 | 0 |
| Metabolism and nutritional disorders | ||||||
| Decreased appetite | 10 | 1 | 6 | 0 | 24 | 2 |
| Infections and infestations | ||||||
| Upper respiratory tract infectionb | 6 | 0 | 3 | 0 | 23 | 1 |
| Pneumoniab | 7 | 7 | 7 | 5 | 21 | 15 |
| Sepsisb | 6 | 6 | 2 | 1 | 14 | 11 |
| Cellulitisb | 4 | 1 | 3 | 2 | 12 | 5 |
| Investigations | ||||||
| Renal impairmentb | 9 | 0 | 8 | 1 | 18 | 0 |
| Weight decreased | 5 | 0 | 3 | 0 | 10 | 1 |
| Injury, poisoning, and procedural complications | ||||||
| Fall | 4 | 0 | 1 | 0 | 12 | 1 |
| Psychiatric disorders | ||||||
| Insomnia | 6 | 0 | 2 | 0 | 12 | 0.5 |
| Vascular disorders | ||||||
| Hypotensionb | 4 | 0 | 6 | 1 | 11 | 2 |
| Cardiac disorders | ||||||
| Arrhythmiab | 3 | 0 | 2 | 0 | 11 | 1 |
aPlease see full Prescribing Information for complete list of adverse events occurring during all cycles.
bIncludes multiple adverse reaction terms.
cIncludes adverse reactions that occurred during all cycles, including during treatment with 1 cycle of intravenous decitabine.
Select hematologic lab abnormalities observed in >20% of the pooled safety population1
| Lab parametera | INQOVI Cycle 1b | IV decitabine Cycle 1b | INQOVI all cyclesb | |||
|---|---|---|---|---|---|---|
| All grades (%) |
Grades 3‑4 (%) |
All grades (%) |
Grades 3‑4 (%) |
All grades (%) |
Grades 3‑4 (%) |
|
| Hematology | ||||||
| Leukocytes decreased | 79 | 65 | 77 | 59 | 87 | 81 |
| Platelet count decreased | 79 | 65 | 77 | 67 | 82 | 76 |
| Neutrophil count decreased | 70 | 65 | 62 | 59 | 73 | 71 |
| Hemoglobin decreased | 58 | 41 | 59 | 36 | 71 | 55 |
aIncludes any lab abnormalities that worsened by ≥1 grades. Grades 3 to 4 include any lab abnormalities that worsened to Grade 3 or Grade 4.
bThe denominator used to calculate the rate varied from 103 to 107 for INQOVI Cycle 1, from 102 to 106 for the IV decitabine cycle, and from 203 to 208 for INQOVI (all cycles) based on the number of patients with a baseline value and ≥1 post-treatment value.
Please see full Prescribing Information for chemistry lab safety parameters.
AML= acute myeloid leukemia; AUC=area under curve; CMML= chronic myelomonocytic leukemia; CR=complete response; HMA=hypomethylating agent; IQR=interquartile range; MDS=myelodysplastic syndromes; NE=not evaluated; NS=not significant; RBC=red blood cell.
References: 1. INQOVI [package insert]. Princeton, NJ: Taiho Oncology, Inc.; 2022. 2. Garcia-Manero G, McCloskey J, Griffiths EA, et al. Oral decitabine-cedazuridine versus intravenous decitabine for myelodysplastic syndromes and chronic myelomonocytic leukaemia (ASCERTAIN): a registrational, randomised, crossover, pharmacokinetics, phase 3 study. Lancet Haematol. 2024;11(1):e15-e26. 3. Savona MR, McCloskey JK, Griffiths EA, et al. Prolonged survival in bi-allelic TP53-mutated (TP53mut) MDS subjects treated with oral decitabine/cedazuridine in the Ascertain trial (ASTX727-02). Blood. 2022;140(suppl 1):2066-2069. 4. Santini V, Stahl M, Sallman DA. TP53 mutations in acute leukemias and myelodysplastic syndromes: insights and treatment updates. Am Soc Clin Oncol Educ Book. 2024;44(3):e432650. 5. Data on file. Taiho Oncology Inc., Princeton, NJ. 6. Kim N, Norsworthy KJ, Subramaniam S, et al. FDA approval summary: decitabine and cedazuridine tablets for myelodysplastic syndromes. Clin Cancer Res. 2022;28(16):3411-3416.
Appropriate Patients
Dosing