Previous Page
Home
Next Page
Appropriate Patients


View a panel discussion between a well-respected hematologist-oncologist, nurse practitioner, and patient caregiver advocate on the topic of myelodysplastic syndromes (MDS) treatment at home—including the advantages and disadvantages of current treatment options, patient care, and INQOVI.
Additional travel to and from chemotherapy infusion centers or hospitals for IV infusions or subcutaneous injections

Venous access and parenteral administration1,5

Of 664 higher-risk patients, 295 (44.4%) were nonpersistent with HMA treatment (nonpersistence is defined by the investigators as <4 cycles or a gap of ≥90 days between cycles)6
](/Content/img/hcp/what-is-inqovi/less-than-four.png)
*Especially in the case of transplant-ineligible patients.
†Surveillance, Epidemiology, and End Results (SEER)-Medicare linked database between 2010 and 2016.
IV=intravenous


INQOVI® tablets are 1 pill, taken once daily for 5 days out of a 28-day cycle. See full dosing information here. INQOVI is a fixed-dose combination of decitabine (35 mg) and cedazuridine (100 mg), a cytidine deaminase inhibitor that enhances oral bioavailability of decitabine and also increases its systemic exposure.
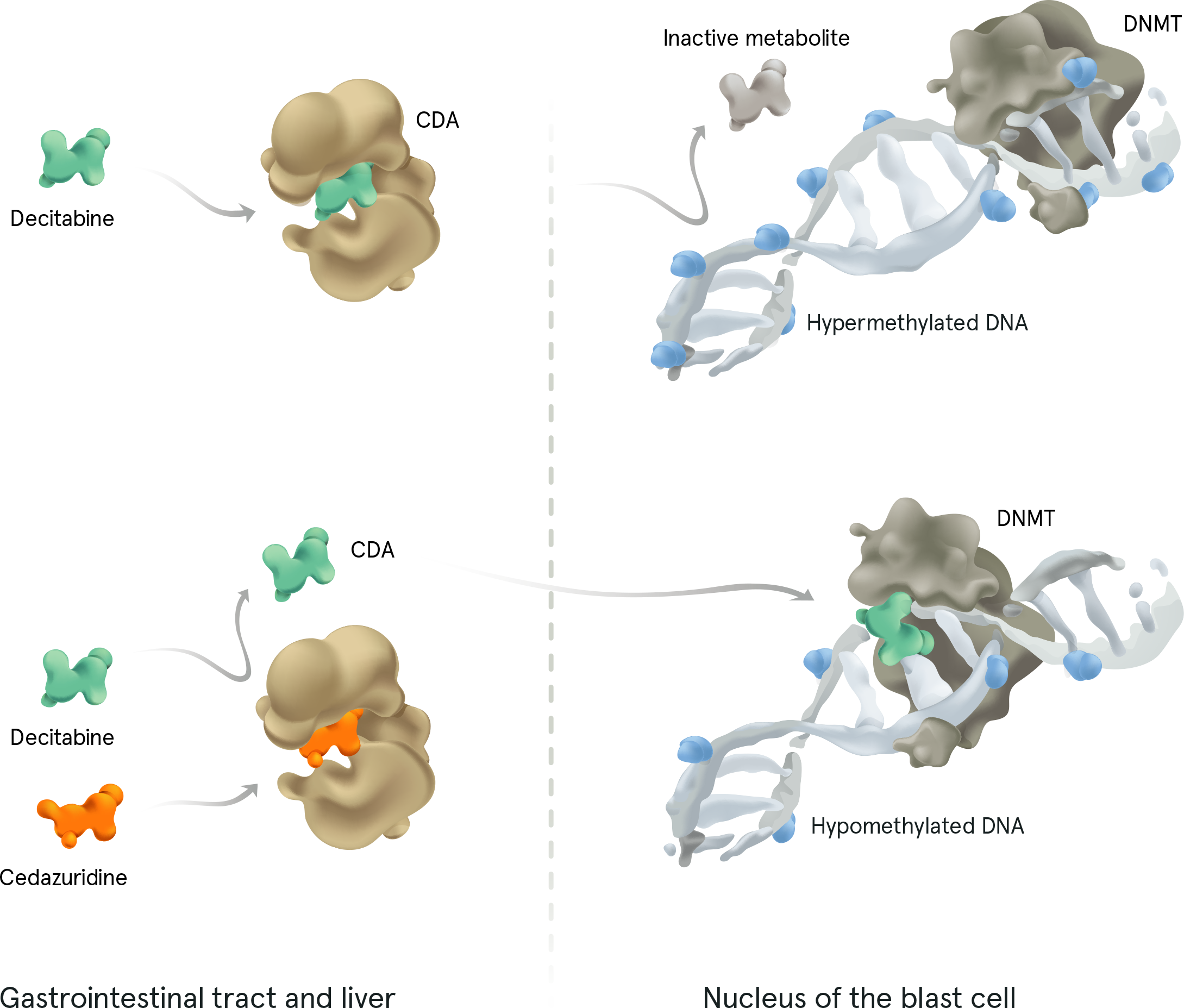
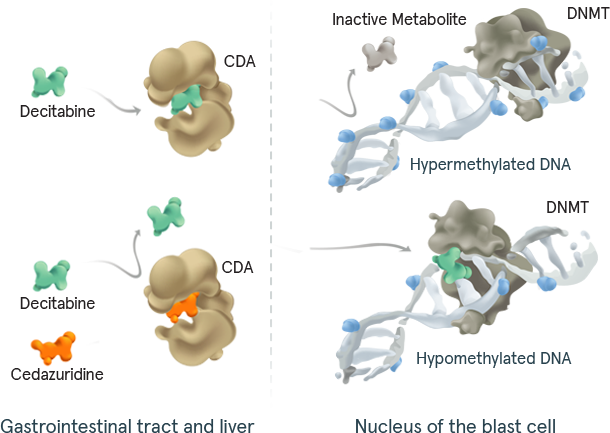
DNMT=DNA methyltransferase.
References: 1. Steensma DP, Komrokji RS, Stone RM, et al. Disparity in perceptions of disease characteristics, treatment effectiveness, and factors influencing treatment adherence between physicians and patients with myelodysplastic syndromes. Cancer. 2014;120(11):1670‑1676. 2. Bell JA, Galaznik A, Blazer M, et al. Economic burden of patients treated for higher‑risk myelodysplastic syndromes (HR‑MDS) in routine clinical care in the United States. PharmacoEconomics Open. 2019;3(2): 237‑245. 3. Savona MR, Odenike O, Amrein PC, et al. An oral fixed‑dose combination of decitabine and cedazuridine in myelodysplastic syndromes: a multicentre, open‑label, dose‑escalation, phase 1 study. Lancet Haematol. 2019;6(4): e194‑e203. doi:10.1016/S2352‑3026(19)30030‑4 4. Vidaza [package insert]. Summit, NJ: Celgene Corporation; 2020. 5. Leveque D. Subcutaneous administration of anticancer agents. Anticancer Research. 2014;34(4):1579‑1586. 6. Joshi N, Kale H, Corman S, et al. Direct medical costs associated with treatment nonpersistence in patients with higher‑risk myelodysplastic syndromes receiving hypomethylating agents: A large retrospective cohort analysis. Clin Lymphoma Myeloma Leuk. 2021;21(3):e248‑e254. 7. Kini V, Ho PM. Interventions to improve medication adherence: A review. JAMA. 2018 Dec 18;320(23):2461‑2473. 8. Platzbecker U. Treatment of MDS. Blood. 2019;133(10):1096‑1107. 9. INQOVI [package insert]. Princeton, NJ: Taiho Oncology, Inc.; 2022
Home
Appropriate Patients